转自:康龙化成
Enhancing Substrate–Metal Catalyst Affinity via Hydrogen Bonding: Pd(II)-Catalyzed β-C(sp3)–H Brominationof Free Carboxylic Acids
Liang Hu, Guangrong Meng, Xiangyang Chen, Joseph S. Yoon, Jing-Ran Shan, Nikita Chekshin, Daniel A. Strassfeld, Tao Sheng, Zhe Zhuang, RodolpheJazzar*, Guy Bertrand*, K. N. Houk*, and Jin-Quan Yu*
Department of Chemistry, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, California 92037, United States; Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, California 90095, United States; UCSD-CNRS Joint Research Laboratory (IRL 3555), Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, California 92093, United States
—J. Am. Chem. Soc.2023, DOI: 10.1021/jacs.3c04223
Recommended by Depei Meng_MC1

ABSTRACT: The achievement of sufficient substrate–metal catalyst affinity is a fundamental challenge for the development of synthetically useful C–H activation reactions of weakly coordinating native substrates. Herein, we report the discovery of a ligand scaffold containing a remote amide motif that can form a favorable meta-macrocyclic hydrogen bonding interaction with the aliphatic acid substrate. The utility of this ligand scaffold is demonstrated through the development of an unprecedented C(sp3)–H bromination of α-tertiary and α-quaternary free carboxylic acids, which proceeds in exceedingly high mono-selectivity. The geometric relationship between the NHAc hydrogen bond donor and the coordinating quinoline ligand is crucial for forming the meta-macrocyclophane-like hydrogen bonding interaction, which provides a guideline for the future design of catalysts employing secondary interactions.
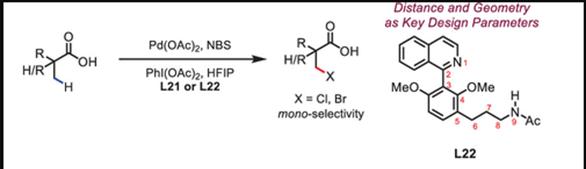

Pd(II)-Catalyzed C(sp3)−H Bromination of Free Aliphatic Acids
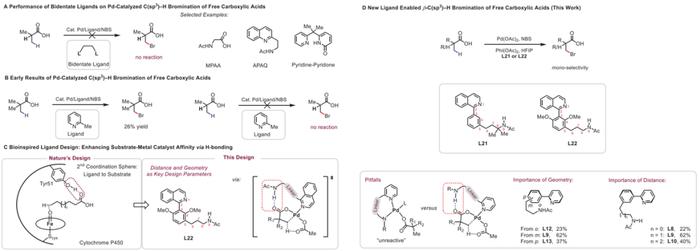

β-C(sp3)−H Bromiantion of Free Aliphatic Acids
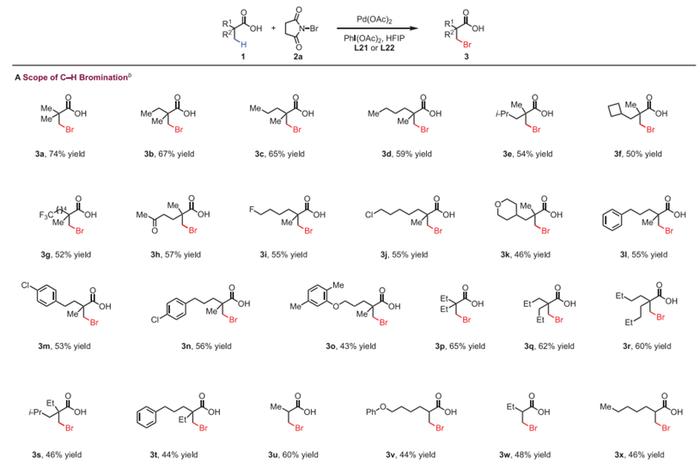

Other Applications
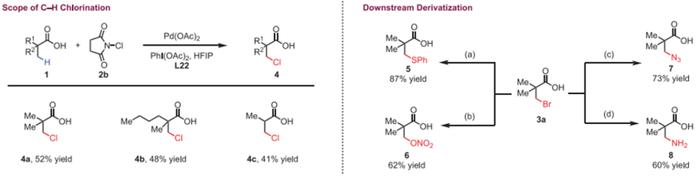

Summary and Comments:
In summary, Prof. Jin-Quan Yu and his co-workers have discovered a new class of pyridine-based ligands containing a hydrogen bond donor that interacts with a carboxyl directing group in substrates, thereby enabling the Pd(II)-catalyzed β-C(sp3)−H bromination and chlorination of free carboxylic acids. The broad substrate scope, as well as the ease of valuable downstream transformations of the halogenated products, demonstrates the synthetic potentiality of this strategy. Importantly, our bioinspired ligand design employing a secondary coordination sphere hydrogen-bonding interaction was the key to the success of this C(sp3)−H halogenation.
总的来说,Jin-Quan Yu教授等发现了一类新的吡啶基配体,该配体可以通过其含有的一个氢键供体与底物中的羧基导向基团相互作用,从而使Pd(II)催化的羧酸β位C(sp3)−H溴化和氯化成为可能。该反应底物范围广,形成的卤代产物易于进行有价值的下游转化,具有很高的合成应用潜力。




举报成功